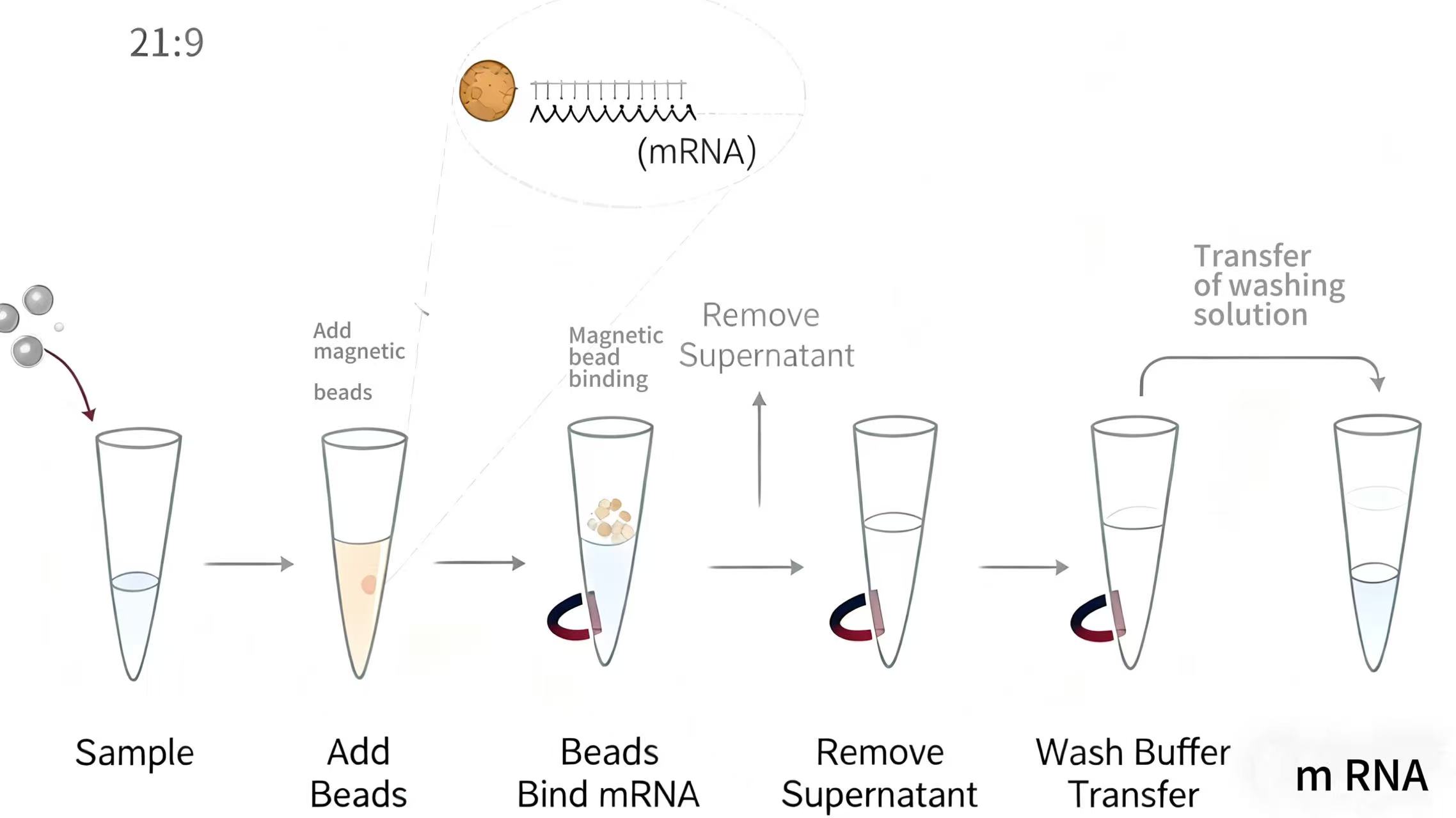
This comprehensive guide identifies frequent errors encountered during magnetic beads DNA extraction and provides practical strategies for avoiding these pitfalls. We will examine mistakes across all process stages, from sample preparation through final elution, and explain how these errors impact DNA yield, quality, and downstream application performance. By understanding both the technical principles and practical implementation of magnetic bead technology, laboratories can optimize their protocols for consistent, reliable results across diverse sample types and applications.
Sample Preparation and Lysis Phase Errors
The initial stages of magnetic beads DNA extraction, including sample preparation and cell lysis, establish the foundation for successful nucleic acid purification. Common mistakes in this phase often involve insufficient sample homogenization, inappropriate sample-to-buffer ratios, or incomplete cell disruption that limit DNA availability for subsequent binding. These errors directly impact final yield and may introduce variability between samples processed using the same protocol.
Proper sample handling before and during lysis ensures complete release of intracellular DNA while maintaining its integrity for downstream applications. Understanding sample-specific requirements for lysis conditions prevents both under-lysis that reduces yield and over-lysis that may promote degradation. Attention to details such as incubation time, temperature, and mixing frequency during this critical phase significantly influences overall extraction success.
Inadequate Sample Homogenization
Incomplete homogenization of tissue samples or insufficient mixing of viscous liquids creates uneven lysis conditions that reduce DNA yield and representativeness. Solid tissues require thorough disruption through mechanical methods such as bead beating or grinding before lysis buffer addition to ensure uniform access to all cellular material. Liquid samples benefit from vigorous vortexing or pipette mixing to distribute cells evenly throughout the solution before lysis initiation.
Incorrect Sample-to-Buffer Volume Ratios
Deviation from recommended sample-to-buffer ratios represents a common error that either dilutes the lysis system excessively or creates overcrowded conditions that impede efficient digestion. Excessive sample volume relative to lysis buffer reduces protease effectiveness and detergent concentration, leading to incomplete protein digestion and DNA release. Conversely, insufficient sample in excessive buffer volume unnecessarily dilutes DNA, potentially pushing concentrations below the efficient binding threshold for magnetic beads.
Improper Lysis Time and Temperature Conditions
Insufficient lysis time or incorrect temperature prevents complete cell disruption and protein degradation, while excessive conditions promote DNA fragmentation and degradation. Different sample types require optimized lysis parameters—blood samples typically need shorter digestion times than tissue or plant materials. Following manufacturer recommendations while adjusting for specific sample characteristics ensures complete lysis without compromising DNA integrity through over-digestion.
Incomplete Inhibition of Nucleases
Failure to adequately suppress nuclease activity during sample preparation and lysis leads to DNA degradation that reduces yield quality and fragment size. Including nuclease inhibitors in collection tubes or initial lysis buffers, working quickly at appropriate temperatures, and ensuring proper sample storage before processing prevents degradation. Particularly challenging samples like animal tissues with high nuclease content may require additional protective measures such as rapid freezing or specialized inhibitor cocktails.
Binding Phase Mistakes and Optimization Strategies
The binding phase represents the core of magnetic beads DNA extraction, where DNA specifically adsorbs to bead surfaces under optimized buffer conditions. Common errors during this stage include incorrect binding buffer composition, inadequate mixing, improper incubation time, or suboptimal bead-to-sample ratios that reduce binding efficiency. These mistakes directly impact DNA recovery and may introduce inconsistencies between extractions.
Understanding the chemistry underlying DNA-bead interactions enables troubleshooting of binding phase issues and protocol optimization for specific sample types. The binding efficiency depends on multiple factors including salt concentration, pH, temperature, and mixing intensity that must be balanced for optimal performance. Attention to these parameters ensures maximum DNA capture while minimizing non-specific binding of contaminants that could affect downstream applications.
Incorrect Binding Buffer Preparation and Storage
Improper preparation of binding buffers, particularly inaccurate salt concentrations or pH levels, significantly reduces DNA adsorption to magnetic beads. Using freshly prepared buffers or properly stored aliquots prevents degradation of sensitive components that facilitate DNA-bead interactions. Regular verification of buffer pH and conductivity ensures consistent performance, while avoiding repeated freeze-thaw cycles preserves buffer integrity for reliable binding efficiency.
Inadequate Mixing During Binding Incubation
Insufficient mixing during the binding phase prevents uniform contact between DNA molecules and bead surfaces, reducing capture efficiency. Continuous or frequent mixing maintains beads in suspension and maximizes interaction opportunities, particularly important for fragmented DNA or low-concentration samples. Overly vigorous mixing, however, may shear high-molecular-weight DNA or create excessive foam that interferes with subsequent separation steps.
Suboptimal Incubation Time and Temperature
Insufficient binding time prevents equilibrium establishment between solution-phase DNA and bead surfaces, while excessive incubation offers diminishing returns and prolongs processing. Most protocols benefit from 5-15 minute binding periods with gentle agitation at room temperature, though specific sample types may require adjustment. Consistent timing across samples ensures comparable binding efficiency and reduces inter-sample variability in final yield.
Improper Bead-to-Sample Ratio
Using incorrect bead quantities relative to sample DNA content either wastes beads through excess capacity or saturates available binding sites, reducing recovery efficiency. Manufacturers typically provide guidelines for bead binding capacity, but samples with unusually high or low DNA concentrations may require adjustment. Preliminary testing with representative samples establishes optimal bead quantities that maximize recovery while maintaining cost-effectiveness.
Washing Phase Errors and Quality Implications
The washing phase removes contaminants while retaining bound DNA on magnetic beads, representing a critical balance between purity preservation and yield maintenance. Common mistakes include insufficient washing that leaves inhibitors behind, excessive washing that elutes bound DNA, or improper buffer formulations that compromise either contaminant removal or DNA retention. These errors directly impact DNA purity and downstream application performance.
Optimized washing protocols efficiently remove proteins, salts, and other impurities without displacing significant amounts of target DNA. Understanding the mechanisms by which different wash buffer components facilitate contaminant removal while stabilizing DNA-bead interactions enables troubleshooting of purity issues. Consistent execution of washing steps, including complete supernatant removal without disturbing the bead pellet, ensures reproducible results across extractions.
Incomplete Supernatant Removal
Leaving residual liquid after washing steps carries forward contaminants into subsequent stages, compromising final DNA purity. Careful aspiration without disturbing the magnetic bead pellet ensures complete wash buffer removal while minimizing bead loss. Angling tubes during separation positions the pellet optimally for supernatant removal, while using appropriately sized pipette tips prevents accidental bead aspiration during liquid removal.
Insufficient or Excessive Washing Cycles
Too few washing cycles leaves inhibitors that interfere with downstream applications, while excessive washing gradually elutes bound DNA, reducing final yield. Most protocols incorporate 2-3 wash steps that balance purity and recovery requirements. Samples with high contaminant loads may benefit from additional washes, while those with minimal impurities might maintain adequate purity with fewer cycles to maximize yield.
Improper Wash Buffer Composition and Preparation
Incorrect ethanol concentration in wash buffers represents a common error that either reduces contaminant removal efficiency or promotes DNA elution during washing. Freshly prepared wash solutions with verified alcohol concentrations maintain consistent performance, while degraded or improperly stored buffers exhibit reduced effectiveness. Including appropriate salts or detergents in wash buffers enhances specific contaminant removal without compromising DNA retention.
Inadequate Drying After Final Wash
Residual ethanol carryover from incomplete drying after the final wash inhibits enzymatic reactions in downstream applications like PCR. Allowing 5-10 minutes for air drying with tube caps open evaporates residual alcohol while preventing bead disintegration from over-drying. Controlled drying at elevated temperatures accelerates the process but risks compromising DNA integrity if improperly monitored.
Elution Phase Mistakes and Yield Optimization
The elution phase releases purified DNA from magnetic beads into an appropriate storage buffer, with common errors including insufficient resuspension, improper buffer selection, suboptimal incubation conditions, or incomplete bead separation. These mistakes reduce final DNA yield and concentration, potentially necessitating sample repetition or resulting in inadequate material for downstream applications.
Effective elution requires disrupting the DNA-bead interactions through altered buffer conditions while maintaining DNA stability and accessibility. Understanding how elution buffer pH, ionic strength, and temperature influence release efficiency enables optimization for different sample types and downstream requirements. Attention to elution volume, incubation time, and mixing intensity maximizes DNA recovery while ensuring the resulting solution meets application-specific concentration needs.
Inadequate Resuspension and Mixing During Elution
Insufficient mixing after elution buffer addition prevents uniform resuspension of magnetic beads, reducing DNA release efficiency. Vigorous vortexing or pipette mixing ensures complete bead dispersal and maximum contact with elution buffer, facilitating efficient DNA dissociation. Extended mixing throughout the elution incubation period further enhances recovery, particularly for high-molecular-weight DNA that may release more slowly from bead surfaces.
Improper Elution Buffer Selection and Preparation
Using incorrect elution buffer pH or composition reduces DNA release efficiency and stability. Low-salt buffers or nuclease-free water at slightly alkaline pH (typically 8.0-8.5) optimally disrupt DNA-bead interactions while maintaining nucleic acid integrity. Fresh preparation and proper storage of elution buffers prevent pH drift or microbial growth that could compromise both elution efficiency and long-term DNA stability.
Suboptimal Elution Incubation Conditions
Insufficient elution time or incorrect temperature reduces DNA release, while excessive conditions offer minimal additional benefit while prolonging processing. Most protocols recommend 2-5 minute elution at elevated temperatures (50-65°C) to enhance dissociation kinetics without promoting degradation. Consistent incubation conditions across samples ensure comparable elution efficiency and reduce inter-sample variability.
Premature Magnetic Separation During Elution
Applying magnetic separation before completing elution incubation prevents full DNA release from beads into solution, reducing final yield. Allowing complete incubation without magnetic influence ensures equilibrium establishment between bead-bound and free DNA. Subsequent magnetic separation should be brief to capture beads while leaving maximum DNA in solution for recovery.
Equipment and Technical Execution Errors
Proper equipment usage and technical execution significantly impact magnetic beads DNA extraction success, with common errors including incorrect magnetic separation, equipment calibration issues, contamination control failures, or protocol deviation. These mistakes introduce variability, reduce efficiency, and potentially compromise result reliability across multiple samples.
Understanding equipment principles and maintaining consistent technique ensures reproducible performance and maximizes the technology's advantages. Regular equipment verification, proper maintenance, and adherence to established protocols prevent technical errors that might otherwise go unrecognized while affecting extraction outcomes. Training personnel in both theoretical principles and practical execution creates a foundation for consistent, high-quality results.
Insufficient Magnetic Separation Efficiency
Incomplete bead capture during separation steps allows bead loss into supernatant, reducing yield and potentially transferring material between samples. Using appropriate magnetic separators with sufficient field strength for specific bead types ensures complete retention during washing and final separation. Proper tube positioning in magnetic racks and adequate separation time facilitates clear supernatant removal without disturbing the bead pellet.
Equipment Calibration and Maintenance Neglect
Failure to regularly calibrate and maintain equipment, particularly automated systems, introduces variability and potential processing errors. Magnetic separators may experience decreased field strength over time, while automated liquid handlers require regular verification of dispensing volumes and tip positioning. Implementing scheduled maintenance and calibration protocols ensures consistent performance and early detection of developing issues.
Contamination Control Procedure Lapses
Inadequate attention to contamination control measures introduces foreign DNA or nucleases that compromise sample integrity and result reliability. Maintaining separate pre- and post-amplification areas, using filter tips, regularly cleaning work surfaces, and implementing negative controls identifies contamination sources. Particular vigilance is required when processing forensic buccal swabs where contamination could have significant consequences.
Excessive Protocol Modification Without Validation
Substantial deviation from validated protocols without proper verification introduces uncharacterized variability and potential processing failures. While optimization for specific sample types is often necessary, changes should be implemented systematically with appropriate controls to assess impact on yield, quality, and reproducibility. Documenting modifications and their outcomes facilitates troubleshooting and protocol refinement.
Quality Assessment and Troubleshooting Approaches
Systematic quality assessment and methodical troubleshooting identify extraction issues before they impact downstream applications, with common mistakes including inadequate quality control measures, improper interpretation of quality metrics, or ineffective corrective actions. Implementing comprehensive quality assurance protocols ensures consistent performance and facilitates continuous process improvement.
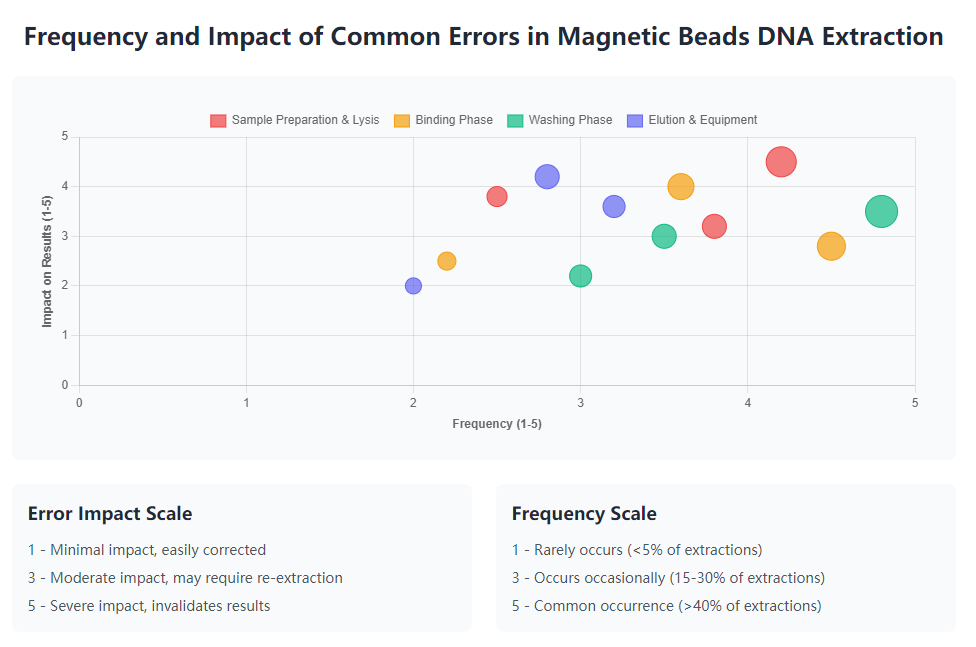
Regular monitoring of key performance indicators including DNA yield, purity ratios, and fragment size distribution detects developing trends that might indicate protocol or reagent issues. Understanding the relationship between specific quality metrics and downstream application requirements enables targeted troubleshooting when results deviate from expectations. Documenting quality data over time establishes performance baselines that aid in distinguishing normal variation from significant problems requiring intervention.
Inadequate Quality Control Implementation
Failure to regularly include control samples in extraction batches prevents detection of systematic issues affecting multiple samples. Implementing positive controls with known DNA quantity and quality, negative controls to monitor contamination, and process controls to verify reagent performance identifies problems before they compromise experimental results. Consistent quality control practices across all extractions provide comparable data for trend analysis and early problem detection.
Misinterpretation of Quality Assessment Results
Incorrect interpretation of spectrophotometric or electrophoretic analysis results leads to inappropriate troubleshooting actions or failure to address genuine issues. Understanding the limitations of different assessment methods prevents over-reliance on single metrics—for example, recognizing that 260/280 ratios alone insufficiently predict PCR performance. Correlating multiple quality metrics with downstream application results establishes meaningful thresholds for acceptable extraction quality.
Ineffective Corrective Action Implementation
Applying generic troubleshooting approaches without identifying root causes often fails to resolve underlying issues, leading to repeated problems. Systematic investigation beginning with reagent verification through process step evaluation identifies specific failure points for targeted correction. Documenting both issues and implemented solutions builds institutional knowledge that streamlines future troubleshooting efforts.
Failure to Monitor Long-Term Performance Trends
Neglecting longitudinal performance tracking misses gradual changes in extraction efficiency that may indicate developing issues with equipment, reagents, or technique. Maintaining quality control databases with key metrics over time facilitates trend identification and proactive intervention before performance degrades significantly. Statistical process control methods can establish acceptable variation ranges that trigger investigation when exceeded.