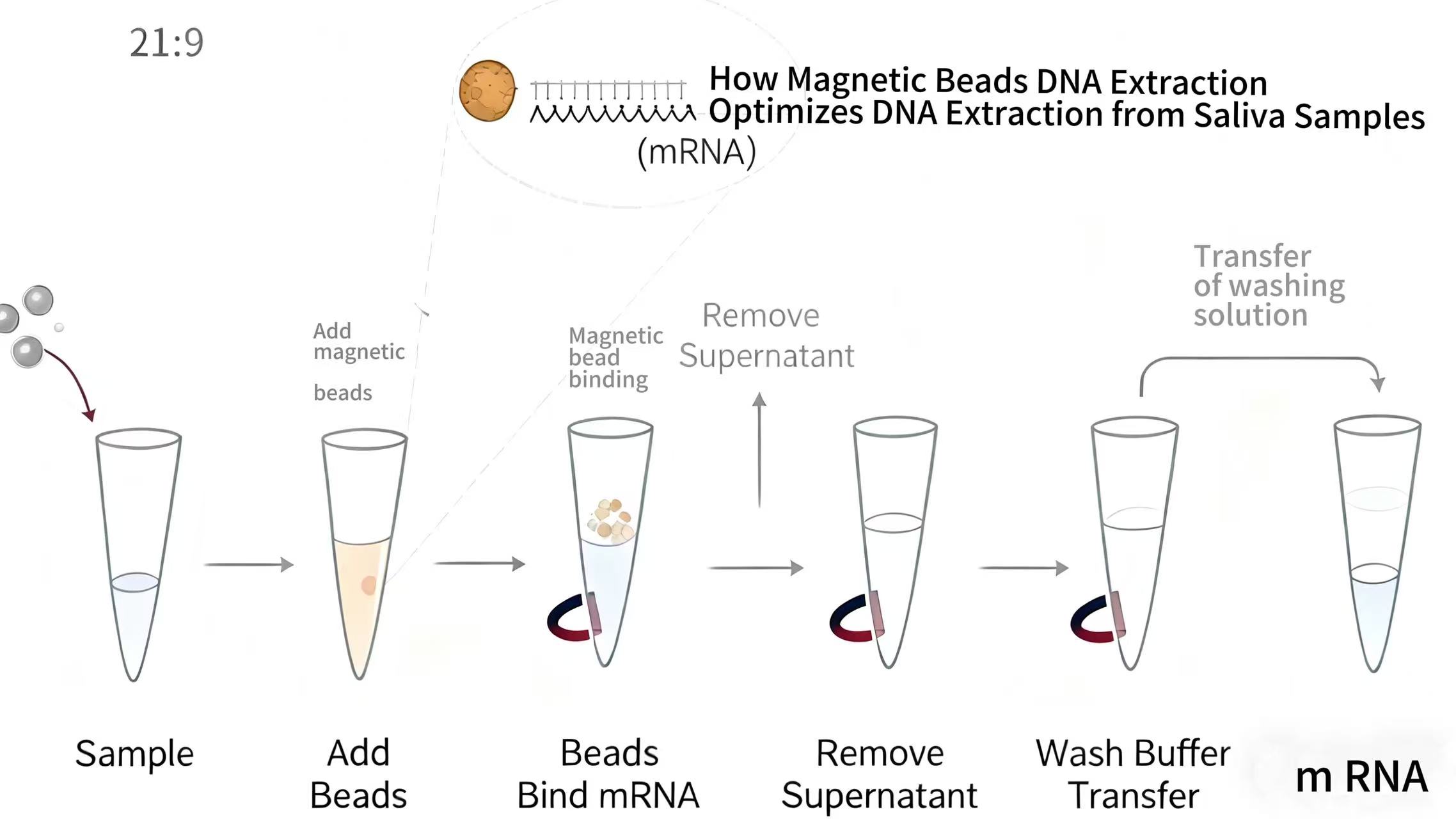
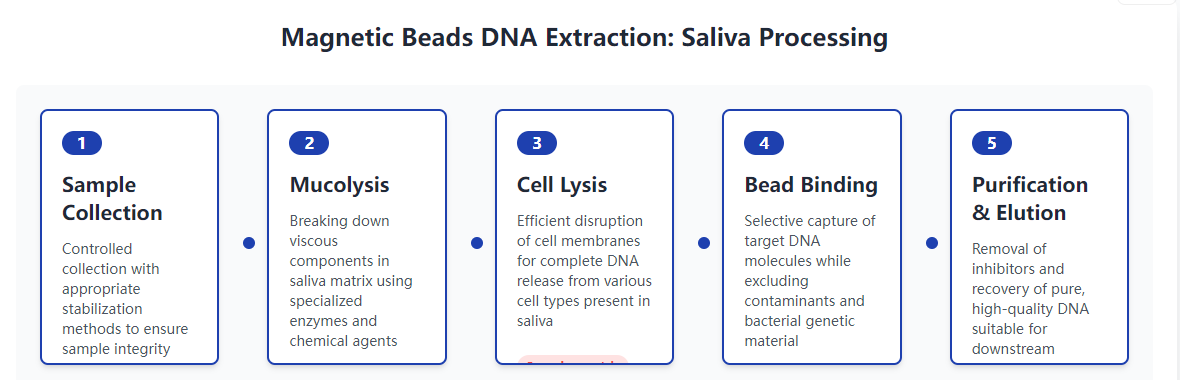
This article examines the specific advantages of magnetic beads DNA extraction technology for processing saliva samples, which present unique challenges including viscosity, bacterial content, and inhibitor presence. We will explore how magnetic bead systems address these issues through specialized protocols, buffer formulations, and processing techniques that maximize DNA yield and quality. From collection to elution, this guide provides comprehensive insights into optimizing saliva DNA extraction for various applications including genetic testing, forensic identification, and epidemiological studies.
Understanding Saliva Sample Characteristics and Challenges
Saliva represents a complex biological fluid containing not only human epithelial cells but also numerous microorganisms, food debris, enzymes, and mucins that complicate DNA extraction. The viscous nature of saliva, primarily due to mucin glycoproteins, creates handling difficulties and can inhibit efficient cell lysis and DNA release. Additionally, the high bacterial load in saliva samples means that extracted DNA often contains significant microbial content that may interfere with human-specific analysis unless properly addressed.
The variable composition of saliva samples based on collection method, time of day, and individual donor characteristics further complicates standardization of extraction protocols. Stimulated saliva collected using commercial devices typically differs in viscosity and cellular content from unstimulated saliva, requiring adjustment of extraction parameters. Understanding these variables is essential for implementing magnetic bead extraction protocols that consistently produce high-quality DNA from diverse saliva sample types.
Complex Matrix Composition and Viscosity Issues
Saliva's complex composition includes mucins, enzymes, electrolytes, and both human and microbial cells that create challenges for efficient DNA extraction. The viscous nature of saliva impedes pipetting accuracy and homogeneous mixing with lysis buffers, potentially leading to incomplete cell lysis and reduced DNA yield. Magnetic bead systems address these issues through vigorous mixing capabilities and specialized lysis conditions that break down mucins and ensure complete sample processing.
High Bacterial Content and Human DNA Selection
The significant microbial population in saliva, typically ranging from 10^8 to 10^9 bacteria per milliliter, means that extraction methods must effectively separate human DNA from microbial genetic material for many applications. Magnetic bead technology can be optimized through buffer conditions and bead surface modifications to preferentially bind human genomic DNA while minimizing co-extraction of bacterial DNA. This selective binding is particularly valuable for genetic testing where human-specific markers are targeted.
Inhibitor Presence and Downstream Interference
Saliva contains various substances that can inhibit downstream molecular applications, including proteases, glycosidases, and food-derived compounds like polyphenols and polysaccharides. These inhibitors can reduce PCR efficiency or completely prevent amplification if not effectively removed during extraction. Magnetic bead systems incorporate specialized wash buffers that specifically target and remove these inhibitory substances while preserving DNA integrity and accessibility.
Sample Stability and Storage Considerations
Saliva DNA begins degrading immediately after collection due to endogenous nuclease activity and bacterial growth, making proper stabilization critical for obtaining high-quality results. Magnetic bead extraction protocols can be adapted to work effectively with various stabilization methods, including commercial preservatives, temperature control, and rapid processing. Understanding how these stabilization approaches interact with magnetic bead chemistry ensures optimal DNA recovery from stored saliva samples.
Magnetic Bead Technology Adaptations for Saliva Samples
Magnetic bead DNA extraction systems have been specifically optimized for saliva samples through modifications to bead characteristics, buffer compositions, and processing parameters. The bead size and surface area have been engineered to maximize binding capacity for the typically lower concentrations of human DNA in saliva while accommodating the high volume often required for sufficient yield. Surface chemistry modifications enhance specificity for human genomic DNA over bacterial DNA and other contaminants.
Buffer systems for saliva extraction include additional mucolytic agents that break down viscous glycoproteins without damaging DNA or interfering with the bead-binding process. These specialized lysis buffers often incorporate reducing agents that break disulfide bonds in mucins alongside proteases that degrade protein complexes that might trap DNA. The optimized salt concentrations in binding buffers ensure efficient DNA capture despite the variable salt content of different saliva samples.
Bead Size and Surface Area Optimization
Magnetic beads designed for saliva processing typically feature smaller diameters and increased surface area to enhance binding capacity for the fragmented DNA often present in these samples. The reduced bead size improves suspension characteristics in viscous saliva lysates, ensuring uniform distribution and maximum contact with target DNA molecules. Surface modifications may include specific functional groups that preferentially interact with human genomic DNA over bacterial or degraded DNA.
Specialized Lysis Buffer Formulations
Saliva-specific lysis buffers incorporate components that address the unique challenges of this sample type, including mucolytic agents like dithiothreitol (DTT) or N-acetylcysteine that break down disulfide bonds in mucin networks. Proteinase K or similar enzymes are included at higher concentrations to efficiently degrade the abundant proteins in saliva that might otherwise co-precipitate with DNA or inhibit binding to magnetic beads. Detergent combinations are optimized to ensure complete cell membrane disruption despite the complex saliva matrix.
Binding Condition Adjustments for Saliva Composition
The binding step in magnetic bead extraction is carefully calibrated for saliva samples to account for their variable pH, salt content, and inhibitor levels. Binding buffer formulations create optimal conditions for DNA adsorption to bead surfaces while minimizing non-specific binding of contaminants. The incubation time and temperature during binding are extended compared to other sample types to ensure maximum recovery from the challenging saliva matrix.
Enhanced Wash Protocols for Inhibitor Removal
Saliva extraction protocols incorporate additional wash steps or modified wash buffer compositions to effectively remove the specific inhibitors prevalent in saliva samples. These enhanced wash protocols may include buffers with slightly different alcohol concentrations or additional detergent components that target the removal of mucin residues, proteases, and bacterial cell wall components. The thoroughness of these washing procedures ensures that extracted DNA is free of substances that could interfere with sensitive downstream applications.
Protocol Optimization for Different Saliva Collection Methods
The method used for saliva collection significantly impacts the optimization of magnetic bead extraction protocols. Unstimulated saliva collected by passive drooling typically has higher viscosity and cellular content than stimulated saliva collected using chewing or chemical stimulants. Commercially available saliva collection devices often include preservatives or stabilizers that must be compatible with the magnetic bead extraction chemistry for optimal results.
Protocol adjustments may include variation in lysis time, buffer volumes, or bead quantities based on the collection method and subsequent sample treatment. Understanding how different collection approaches affect sample characteristics enables laboratories to customize magnetic bead extraction parameters for consistent performance across diverse sample types. This flexibility makes magnetic bead technology suitable for studies utilizing multiple collection methods or historical samples with unknown collection details.
Unstimulated Saliva Collection and Processing
Unstimulated saliva samples typically contain higher concentrations of epithelial cells and DNA but present greater challenges due to increased viscosity. Magnetic bead protocols for these samples often begin with extended lysis times and additional vortexing steps to ensure complete homogenization before proceeding to the binding phase. The higher cellular content may necessitate increased bead quantities or binding buffer volumes to accommodate the greater DNA load while maintaining efficient recovery.
Stimulated Saliva and Commercial Collection Devices
Stimulated saliva collected using commercial devices often has reduced viscosity but may contain chemical stimulants or preservatives that affect extraction efficiency. Magnetic bead protocols must be validated for compatibility with these additives, which might include citric acid, chewing stimulants, or antibacterial agents. Some collection systems incorporate specialized reagents designed specifically for magnetic bead extraction from saliva, streamlining the process and ensuring optimal results.
Oral Swab and Buccal Cell Collection Methods
Buccal cell collection using swabs or brushes represents another common approach for obtaining saliva-derived DNA samples. Magnetic bead extraction efficiently processes these samples by combining swab elution with direct lysate processing without additional transfer steps. The protocol may include mechanical disruption through vortexing with beads or specialized swab processing tubes that maximize cell release from the collection medium before proceeding with standard magnetic separation steps.
Historical and Archived Saliva Samples
Archived saliva samples stored long-term under various conditions present unique challenges including DNA degradation and potential inhibitor formation. Magnetic bead extraction protocols can be modified for these samples through extended lysis, additional purification steps, or adjusted binding conditions that accommodate degraded DNA. The technology's flexibility allows optimization for recovering usable DNA from valuable historical collections that might be incompatible with other extraction methods.
Downstream Applications and Quality Requirements
The quality of DNA extracted from saliva using magnetic bead technology directly impacts its performance in various downstream applications. Genetic testing requiring single nucleotide polymorphism (SNP) analysis or sequencing demands high-molecular-weight DNA with minimal fragmentation, which can be achieved through optimized saliva extraction protocols. Forensic applications utilizing short tandem repeat (STR) profiling require DNA free of inhibitors that might interfere with amplification, a key strength of magnetic bead purification.
Different downstream applications have varying requirements for DNA concentration, purity, and fragment size that influence how saliva extraction protocols should be optimized. Magnetic bead systems provide the flexibility to adjust elution volumes and purification stringency to meet these specific needs. Understanding these application requirements ensures that extracted saliva DNA performs reliably in subsequent analysis, whether for research, diagnostic, or identification purposes.
Genetic Testing and Sequencing Applications
Next-generation sequencing and array-based genetic testing require high-quality DNA with minimal contamination and optimal fragment size distribution. Magnetic bead extraction from saliva consistently produces DNA suitable for these applications through careful control of lysis conditions and minimal mechanical disruption that might fragment DNA. The efficient inhibitor removal ensures high sequencing library preparation efficiency and accurate variant calling in genetic analysis.
Forensic Identification and STR Profiling
Forensic DNA analysis from saliva samples demands complete removal of PCR inhibitors and consistent yield for reliable STR amplification. Magnetic bead technology excels in this application through stringent washing protocols that eliminate substances like hematin, calcium ions, and polysaccharides that can inhibit DNA polymerase activity. The method's reproducibility ensures consistent results across different samples and operators, essential for forensic credibility.
Epidemiological Studies and Biobanking
Large-scale population studies and biobanking applications process thousands of saliva samples requiring standardized, high-throughput extraction methods. Magnetic bead systems provide the necessary scalability and consistency for these endeavors while maintaining sample integrity throughout processing. The technology's compatibility with automation enables efficient processing of large sample batches with minimal hands-on time while ensuring uniform quality suitable for long-term storage and diverse future applications.
Microbiome Analysis and Metagenomic Studies
Saliva microbiome research requires careful balancing of human and microbial DNA recovery based on study objectives. Magnetic bead extraction can be tuned through binding condition adjustments to preferentially isolate either human or microbial DNA, or to comprehensively recover total DNA from saliva. This flexibility supports diverse research approaches, from human genetic analysis to characterization of the oral microbiome using the same initial sample material.
Troubleshooting Common Saliva Extraction Issues
Despite the advantages of magnetic bead technology for saliva DNA extraction, certain issues may arise that require troubleshooting for optimal results. Low DNA yield often results from incomplete cell lysis or suboptimal binding conditions, while poor DNA quality typically indicates insufficient inhibitor removal or DNA degradation during processing. Understanding how to identify and address these issues ensures consistent success with saliva samples across different collection methods and storage conditions.
Systematic troubleshooting approaches should examine each step of the extraction process, from sample reception through final elution, to identify potential points of failure. Method validation using control samples and regular quality assessment of extracted DNA provides early detection of issues before they impact experimental results. Implementing corrective actions based on understanding of both saliva chemistry and magnetic bead principles resolves most common extraction problems effectively.
Addressing Low DNA Yield and Recovery
Insufficient DNA yield from saliva samples may result from inadequate lysis, insufficient binding capacity, or incomplete elution. Troubleshooting should begin with verification of lysis efficiency through microscopic examination or protein quantification before proceeding to DNA binding. Increasing lysis time, optimizing protease concentration, or ensuring proper mixing during binding can significantly improve yield. For samples with exceptionally low cellular content, increasing starting volume or concentrating the sample before extraction may be necessary.
Improving DNA Purity and Removing Inhibitors
Contaminants co-extracted with DNA from saliva can inhibit downstream applications, manifested as reduced PCR efficiency or complete amplification failure. Enhancing purity typically involves optimizing wash steps through increased wash volumes, additional wash repetitions, or modified wash buffer compositions. Incorporating specific inhibitor removal reagents or extending wash incubation times often resolves purity issues without significantly impacting DNA yield.
Managing Viscosity and Handling Difficulties
Highly viscous saliva samples can challenge pipetting accuracy and homogeneous mixing with reagents, leading to inconsistent results. Pre-treatment with mucolytic agents before formal lysis, increased vortexing during processing, or sample dilution can reduce viscosity-related issues. Automated liquid handling systems specifically programmed for viscous fluids provide an alternative approach for laboratories processing large numbers of challenging saliva samples.
Preventing DNA Degradation During Processing
DNA degradation in saliva extracts evident as smeared electrophoretic patterns or poor performance in applications requiring long amplicons typically results from nuclease activity or mechanical shearing. Adding nuclease inhibitors to collection devices or lysis buffers, reducing processing times, and minimizing vigorous pipetting can preserve DNA integrity. Ensuring proper sample storage before extraction and working quickly once samples are lysed also helps maintain high-molecular-weight DNA.
Comparative Analysis with Other Extraction Methods
When evaluated against alternative DNA extraction technologies for saliva samples, magnetic bead systems demonstrate distinct advantages in yield, quality, and processing efficiency. Traditional phenol-chloroform extraction, while effective for difficult samples, involves hazardous chemicals and multiple transfer steps that increase contamination risk and processing time. Column-based methods provide reasonable results but often struggle with the viscous nature of saliva and may clog during processing, leading to inconsistent recovery.
Direct PCR approaches that bypass extraction entirely work for some saliva applications but provide no control over DNA concentration or purity and remain vulnerable to inhibition. Magnetic bead technology strikes an optimal balance between simplicity and effectiveness, providing purified, concentrated DNA suitable for diverse applications while maintaining efficiency and scalability. This positions magnetic bead extraction as the preferred method for many laboratories processing saliva samples across various fields.
Comparison with Column-Based Extraction Methods
Spin column systems frequently encounter challenges with saliva samples due to filter clogging from mucins and other viscous components, leading to variable recovery and extended processing times. Magnetic bead technology avoids these issues through a filter-free approach that maintains consistent fluid flow and binding efficiency regardless of sample viscosity. The elimination of centrifugation steps in magnetic systems also reduces hands-on time and enables parallel processing of multiple samples.
Evaluation Against Traditional Organic Extraction
Phenol-chloroform extraction, while capable of processing challenging saliva samples, involves significant safety concerns and requires considerable technical expertise for consistent results. Magnetic bead systems provide comparable or superior DNA quality without hazardous chemical handling, making them suitable for routine laboratory use by personnel with varying experience levels. The closed-tube nature of magnetic extraction additionally reduces environmental contamination concerns associated with organic solvent disposal.
Assessment of Direct PCR Approaches
Direct amplification from saliva without DNA extraction works for some applications but offers no control over DNA concentration and remains highly vulnerable to PCR inhibitors present in saliva. Magnetic bead extraction provides purified DNA at controlled concentrations, enabling quantitative analysis and standardization across samples. The ability to store extracted DNA for future analysis represents another significant advantage over direct PCR approaches for valuable samples.
Cost-Benefit Analysis for Different Laboratory Settings
The economic evaluation of magnetic bead technology for saliva DNA extraction must consider both reagent costs and labor requirements across different laboratory scales. While per-sample costs may be slightly higher than some alternative methods, the reduced hands-on time, higher success rates, and better downstream performance often justify the investment. For high-throughput laboratories, the compatibility with automation provides additional economic advantages through increased processing capacity and reduced personnel requirements.