The elution step in DNA extraction might seem like a simple final act, but it holds the key to determining both the quantity and quality of your final DNA yield. This crucial phase involves releasing the purified DNA from its binding matrix into a solution where it can be stored and used for downstream applications. Many researchers experience disappointing DNA concentrations not because of problems during lysis or binding, but due to suboptimal elution conditions. Understanding the science behind elution and implementing strategic optimizations can significantly increase your DNA recovery, saving both time and valuable samples.
Optimizing elution is particularly important when working with limited or precious samples, such as in forensic analysis or when extracting from challenging sources like plant tissues or clinical specimens. The goal is to maximize the amount of DNA that transitions from the solid phase (the membrane or beads) to the liquid phase (your collection tube) while maintaining its integrity and purity. Several factors influence this process, including buffer composition, temperature, contact time, and technique, each of which can be fine-tuned to match your specific requirements.
Understanding the Science Behind DNA Elution
DNA elution operates on fundamental biochemical principles that govern the interaction between DNA molecules and the silica-based surfaces found in most extraction kits. During the binding phase, chaotropic salts create conditions where water molecules are disrupted, allowing DNA to form hydrogen bonds with silica. The elution process reverses this relationship by reintroducing an aqueous environment where DNA molecules prefer to interact with water rather than with the solid silica surface.
The efficiency of this separation depends on breaking the specific interactions that hold DNA to the binding matrix while ensuring the DNA remains stable in solution. This delicate balance requires careful consideration of the chemical environment, as DNA can become damaged or degraded if conditions become too extreme. Understanding these underlying mechanisms provides the foundation for making informed decisions about how to modify standard protocols to improve DNA recovery across different sample types and extraction methods, including spin column systems and magnetic bead technologies.
The Role of pH and Ionic Strength in DNA Release
The pH of your elution buffer plays a critical role in determining how effectively DNA is released from the binding matrix. Most commercial kits recommend using elution buffers with a slightly alkaline pH, typically between 8.0 and 8.5. At this pH range, the phosphate groups in the DNA backbone become increasingly negatively charged, strengthening the electrostatic repulsion between the DNA and the negatively charged silica surface. This repulsive force helps to push the DNA molecules away from the binding matrix and into solution.
Ionic strength similarly influences the elution efficiency by modulating the electrostatic interactions between DNA and the silica surface. Low ionic strength environments, such as those created by Tris-EDTA buffer or nuclease-free water, reduce the shielding of charges on both surfaces, increasing their mutual repulsion and promoting DNA release. However, extremely low ionic strength can sometimes lead to DNA degradation over time, which is why many researchers prefer a balanced approach using a weak salt solution that provides both efficient elution and long-term stability for the extracted DNA.
Strategic Optimization of Elution Conditions
Moving beyond basic understanding to practical application requires systematically addressing the key variables that influence elution efficiency. The most significant factors include elution buffer selection, temperature manipulation, incubation timing, and technical execution. Each of these elements can be independently optimized, though they often work synergistically to improve overall DNA recovery. The optimal combination may vary depending on your specific extraction kit, sample type, and intended downstream applications.
Researchers working with particularly challenging samples, such as animal tissues or environmental samples, often benefit most from these optimization strategies. Even small improvements in elution efficiency can make the difference between having sufficient DNA for analysis and needing to repeat extractions, particularly when sample material is limited or difficult to obtain. The following sections provide evidence-based approaches to maximizing your DNA yield through targeted elution optimization.
Selecting the Optimal Elution Buffer
Choosing the right elution buffer represents one of the most impactful decisions in optimizing DNA concentration. While most commercial kits provide a specific elution buffer, researchers can often achieve better results by testing alternatives. Tris-EDTA (TE) buffer at pH 8.0 remains the gold standard for long-term DNA storage, as the Tris component maintains a stable pH while EDTA chelates magnesium ions that would otherwise activate DNase enzymes. For immediate use in downstream applications, nuclease-free water may be preferable as it introduces no additional salts or chemicals that might interfere with enzymatic reactions.
The volume of elution buffer used significantly affects final DNA concentration. Using a smaller volume will yield a more concentrated DNA solution, but may sacrifice overall recovery if insufficient to fully hydrate and release all bound DNA. A common strategy involves performing two sequential elutions with small volumes rather than a single elution with a larger volume. The first elution typically recovers 60-80% of the bound DNA, while the second collects most of the remainder, allowing researchers to either combine them for maximum total yield or keep them separate, with the first being more concentrated. This approach is particularly valuable when working with silica bead systems where complete resuspension can be challenging.
Temperature and Incubation Time Optimization
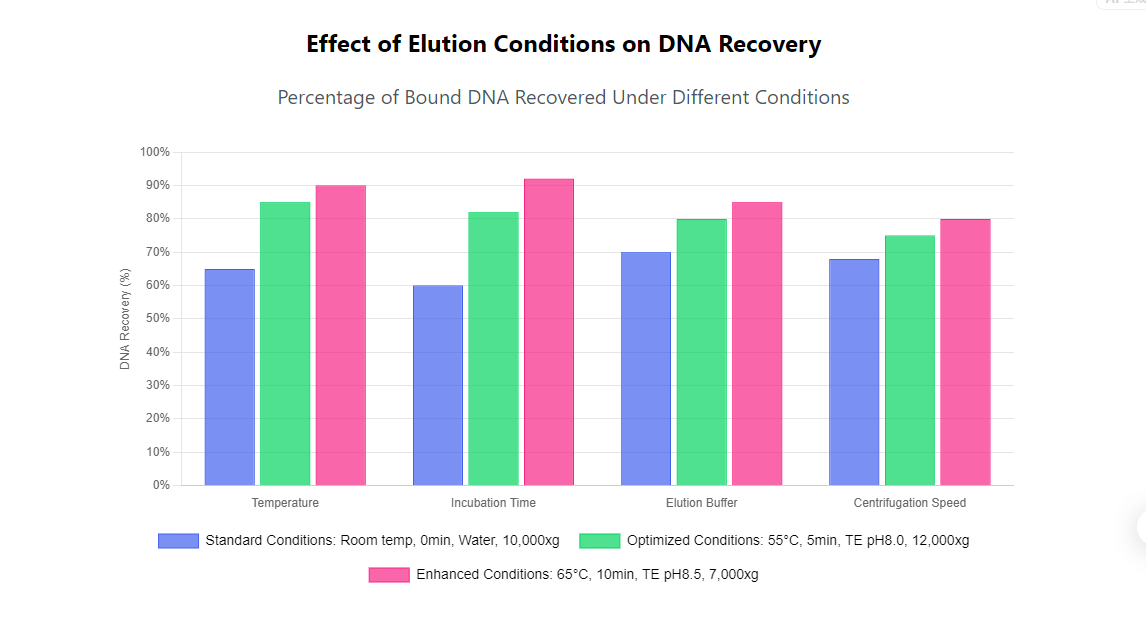
Temperature manipulation during elution can dramatically improve DNA yield by increasing molecular motion and weakening the hydrogen bonds between DNA and the silica matrix. Most protocols benefit from pre-warming the elution buffer to 55-65°C before application to the membrane or beads. This elevated temperature helps to overcome the energy barrier for DNA release without causing significant denaturation or degradation of the DNA molecules. The optimal temperature may vary slightly between different kit formulations, with some systems performing best at the lower end of this range while others benefit from higher temperatures.
Incubation time represents another critical variable that is often overlooked in standard protocols. While many quick extraction methods suggest immediate centrifugation after adding elution buffer, allowing a 5-10 minute incubation period before centrifugation can increase DNA yield by 20-50%. During this time, the elution buffer fully hydrates the binding matrix and establishes equilibrium conditions that favor DNA dissolution. For difficult samples or when maximum recovery is essential, extending this incubation to 15-30 minutes may provide additional benefits, though diminished returns typically set in beyond this point. This extended incubation is particularly helpful when dealing with high molecular weight DNA or when using rapid extraction kits that employ faster-binding chemistry.
Technical Execution for Maximum Recovery
Beyond chemical and temperature considerations, the physical execution of the elution step can significantly impact DNA concentration. Proper technique ensures that the elution buffer makes complete contact with all DNA-binding surfaces and that the released DNA is efficiently transferred to the collection tube. Small adjustments to standard protocols can yield substantial improvements in recovery, particularly when working with small elution volumes where losses become more significant proportionally.
These technical considerations become increasingly important when processing multiple samples simultaneously, as consistency across samples ensures comparable results in downstream applications. Researchers should establish standardized protocols that account for these technical factors and train all laboratory personnel in their consistent application. This attention to technical detail often separates successful DNA extraction outcomes from disappointing ones, especially in demanding applications such as food testing or research applications requiring high-quality DNA.
Proper Membrane Hydration and Centrifugation
Effective elution requires complete hydration of the silica membrane or bead matrix to ensure the elution buffer contacts all bound DNA. When adding elution buffer to spin columns, it should be applied directly to the center of the membrane and allowed to spread evenly across the surface before centrifugation. For bead-based systems, thorough resuspension of the pellet during elution ensures maximum surface area exposure to the buffer. Incomplete hydration leaves pockets of DNA bound to the matrix that never encounter the elution buffer, resulting in unnecessary losses.
Centrifugation speed and duration also influence elution efficiency. Most protocols recommend standard centrifugation speeds (10,000-14,000 x g) for 1 minute, but some evidence suggests that slower centrifugation (5,000-7,000 x g) for longer durations (2-3 minutes) may improve DNA recovery by allowing more time for diffusion and elution buffer interaction with the matrix. Additionally, a second brief centrifugation of the same collection tube after elution can recover additional liquid that remains in the column or tube caps, typically adding 5-10% to the final volume and consequently increasing the total DNA yield.
Troubleshooting Common Elution Problems
Even with careful optimization, researchers sometimes encounter challenges during the elution step that result in lower-than-expected DNA concentrations. Systematic troubleshooting of these issues can identify the underlying causes and guide appropriate corrective actions. Common problems include incomplete DNA release, degradation of eluted DNA, and contamination with inhibitors that affect downstream applications. Each of these issues has distinct signatures and solutions that experienced researchers learn to recognize and address.
Documenting elution problems and their solutions creates valuable institutional knowledge that improves laboratory efficiency over time. Maintaining detailed records of extraction conditions, including lot numbers of reagents, specific equipment used, and any deviations from standard protocols, facilitates faster troubleshooting when problems arise. This systematic approach to problem-solving is especially valuable in regulated environments where consistency and documentation are essential requirements for valid results.
Addressing Low Yield and Poor Quality Issues
When DNA concentrations consistently fall below expectations, the problem may trace back to incomplete washing during previous steps. Residual ethanol from wash buffers can interfere with elution by preventing DNA from dissolving properly in the aqueous elution buffer. Ensuring complete evaporation of ethanol during the drying step (typically 5 minutes at room temperature with open lids) before elution can significantly improve yields. Conversely, excessive drying can make DNA more difficult to elute, particularly in low-humidity environments, suggesting an optimal balance must be found.
Poor DNA quality after elution often manifests as degraded samples or the presence of inhibitors that affect downstream applications. Degradation can result from using elution buffers that are too alkaline (pH >9.0) or from excessively high elution temperatures. The presence of inhibitors often indicates incomplete washing, as contaminants carried over from earlier steps become concentrated in the small elution volume. Re-optimizing wash steps and ensuring proper buffer removal before proceeding to elution typically resolves these quality issues. For particularly challenging samples, such as those containing high levels of polyphenols or polysaccharides, specialized extraction methods may be necessary before standard kit-based purification.
Validating and Measuring Optimization Success
Implementing elution optimizations requires validation to ensure they actually improve outcomes without introducing new problems. The most direct validation method involves comparing DNA concentrations and quality before and after implementing changes, using standardized measurement techniques. Spectrophotometric methods like Nanodrop measurements provide information about both concentration and purity through A260/A280 and A260/A230 ratios, while fluorometric methods like Qubit offer more accurate concentration measurements specifically for DNA.
Beyond simple concentration measurements, functional validation through downstream applications provides the ultimate test of optimization success. PCR amplification efficiency, restriction enzyme digestion patterns, and sequencing library quality all reflect the practical utility of the extracted DNA. Successful optimization should improve not just the concentration reading but also the performance of the DNA in its intended applications. This comprehensive approach to validation ensures that optimizations deliver meaningful improvements rather than just numerical changes that don't translate to better experimental outcomes.
Establishing Quality Control Metrics
Implementing consistent quality control measures allows researchers to track elution efficiency over time and identify trends or deviations from expected performance. Basic metrics include recording the DNA concentration, volume recovered, and purity ratios for each extraction. More advanced quality control might involve running a small aliquot on an agarose gel to visualize DNA integrity or performing a standardized PCR amplification to confirm functionality. These metrics create a baseline against which optimization attempts can be objectively evaluated.
Laboratories processing similar sample types regularly should establish expected yield ranges for different sample inputs. Significant deviations from these expected ranges signal potential problems with either the extraction process or the samples themselves. This proactive approach to quality control helps identify issues before they compromise experimental results, saving both time and resources. When consistent problems are identified, methodical testing of individual variables in the elution process can pinpoint the specific factors needing adjustment, leading to targeted solutions rather than guesswork.
```