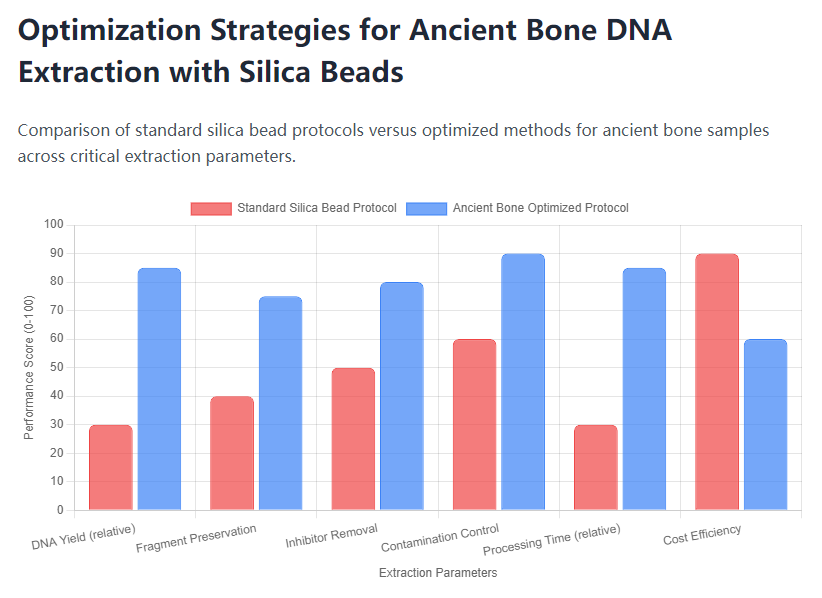
Ancient bone DNA extraction represents one of the most demanding applications in molecular archaeology and paleogenetics, requiring specialized approaches to overcome the substantial degradation and contamination challenges inherent in aged skeletal remains. Silica bead-based DNA extraction kits have emerged as powerful tools for these investigations, though their application requires careful modification of standard protocols to address the unique characteristics of ancient skeletal material. The successful recovery of genetic information from archaeological contexts depends on understanding both the limitations of ancient DNA preservation and the biochemical principles underlying silica-based purification methods. This comprehensive examination addresses the principal challenges encountered when working with ancient bone specimens and outlines evidence-based strategies for optimizing silica bead extraction protocols to maximize DNA yield and quality while minimizing contamination risks.
Fundamental Characteristics of Ancient Bone DNA
Ancient DNA molecules extracted from bone tissue undergo extensive post-mortem damage including fragmentation, deamination, and cross-linking that fundamentally differentiates them from modern DNA samples. The average fragment length in well-preserved ancient bone rarely exceeds 100-200 base pairs, with the majority of molecules measuring between 30-80 base pairs depending on environmental conditions and specimen age. Hydrolytic damage cleaves phosphodiester bonds while oxidative damage creates blocking lesions that prevent polymerase extension during amplification. These molecular alterations necessitate specialized extraction and analysis approaches distinct from those used for contemporary samples. The extreme fragmentation particularly impacts silica bead binding efficiency, as shorter DNA fragments demonstrate reduced affinity for silica surfaces under standard binding conditions.
Environmental factors including temperature, moisture, pH, and microbial activity significantly influence DNA preservation in bone tissue through their effects on degradation kinetics. Cool, dry, and stable environments generally favor preservation, while fluctuating conditions with repeated wet-dry cycles accelerate DNA degradation. The mineral component of bone provides some protection against enzymatic degradation through the formation of hydroxyapatite-DNA complexes, though this same mineral matrix complicates DNA extraction by binding nucleic acids and inhibiting their release during standard lysis procedures. The complex interplay between bone mineral content, collagen preservation, and DNA survival creates substantial variability in extraction success even among specimens from the same archaeological context, necessitating flexible protocols adaptable to individual specimen characteristics.
Contamination Challenges in Ancient Bone Studies
Modern DNA contamination represents perhaps the most significant challenge in ancient bone studies, as minuscule quantities of contemporary DNA can overwhelm the degraded endogenous ancient DNA signal. Contamination sources include archaeological excavators, laboratory personnel, and previous handling of specimens, with bone powdering procedures particularly prone to introducing exogenous DNA. The porous structure of bone tissue readily absorbs environmental contaminants that become integrated into the matrix over time, complicating decontamination procedures. Unlike modern forensic samples where the contributor profile is unknown, ancient studies face the additional challenge that potential contaminants may share genetic ancestry with the ancient individual, making contamination detection through standard genetic distance measures less reliable.
Rigorous laboratory procedures following ancient DNA dedicated facility guidelines provide the foundation for contamination control, including physical separation of pre-and post-PCR activities, unidirectional workflow, and extensive environmental cleaning. Surface decontamination of bone specimens typically involves mechanical removal of the outer surface followed by chemical treatment with sodium hypochlorite or exposure to UV radiation, though these procedures must balance contamination removal against potential destruction of surviving endogenous DNA. The implementation of forensic bone extraction protocols adapted for ancient specimens incorporates blank controls at multiple processing stages to monitor contamination introduction throughout the workflow. These controls become particularly important when analyzing specimens from populations with known genetic affinities to potential laboratory personnel.
Silica Bead Chemistry Modifications for Ancient DNA
Standard silica bead DNA binding conditions optimized for modern DNA perform suboptimally with ancient specimens due to the shorter fragment length and chemical modifications characteristic of degraded DNA. The binding affinity between DNA and silica surfaces depends on multiple factors including fragment length, with molecules shorter than 100 base pairs demonstrating significantly reduced binding efficiency under standard high-salt conditions. Ancient DNA protocols frequently modify binding buffer composition to include higher concentrations of chaotropic salts, typically guanidinium thiocyanate at concentrations up to 5M compared to the 4M used for modern DNA extraction. These enhanced chaotropic conditions improve binding of shorter fragments by more completely disrupting the hydration shell surrounding DNA molecules, though they may also increase co-purification of inhibitors if not carefully balanced with washing efficiency.
Binding incubation time and temperature require optimization for ancient bone extracts, with extended incubation periods at elevated temperatures sometimes improving recovery of particularly degraded templates. The ratio of silica beads to sample volume often benefits from adjustment, with increased bead quantities sometimes necessary to capture the relatively low mass of highly fragmented ancient DNA. However, excessive bead quantities can increase non-specific binding of inhibitors and reduce final DNA purity. The silica bead size distribution itself influences binding efficiency, with smaller diameter beads (1-5μm) theoretically providing greater surface area for fragment binding though potentially complicating subsequent separation steps. The optimal silica bead parameters for bone extraction must be determined empirically for specific specimen types and preservation conditions.
Bone Demineralization and Digestion Strategies
Efficient DNA recovery from ancient bone requires complete demineralization to release DNA molecules bound within the hydroxyapatite matrix, a process that standard lysis buffers designed for soft tissues accomplish incompletely. Ancient DNA protocols typically incorporate an extended demineralization step using EDTA at concentrations of 0.5M and pH 8.0, with incubation periods ranging from 24 hours to several days depending on bone density and preservation. Complete demineralization is evidenced by complete softening of bone powder, though the extended incubation increases opportunities for contemporary DNA contamination and potential DNA degradation if conditions are not carefully controlled. The demineralization step precedes proteinase K digestion, which then operates more efficiently on the collagen matrix once mineral components have been removed.
Proteinase K concentration and digestion time require substantial increase for ancient bone compared to modern specimens, with typical protocols using 1-2 mg/mL enzyme concentrations and digestion periods of 24-48 hours at 56°C. Some particularly recalcitrant specimens benefit from the addition of complementary proteases or digestion at higher temperatures using thermostable proteinase K variants, though elevated temperatures may accelerate hydrolytic damage to already fragile DNA molecules. The inclusion of reducing agents such as DTT helps break down disulfide bonds in preserved collagen, while detergents like Sarkosyl improve membrane disruption and inhibitor solubilization. These modifications to standard silica bead kit lysis procedures dramatically improve DNA release from ancient bone while maintaining compatibility with subsequent silica binding steps.
Inhibitor Removal and Purity Optimization
Ancient bone extracts typically contain multiple classes of PCR inhibitors including humic substances, calcium ions, collagen derivatives, and Maillard reaction products that persist through standard silica bead washing procedures. These compounds interfere with downstream enzymatic reactions through diverse mechanisms including polymerase inhibition, DNA binding competition, and enzyme cofactor chelation. Silica bead protocols modified for ancient bone often incorporate additional washing steps with specialized buffers containing chelating agents, organic solvents, or competitor molecules that displace inhibitors from binding sites without eluting the target DNA. The optimal washing regimen must be determined empirically for specific archaeological contexts, as inhibitor profiles vary substantially based on depositional environment.
Humic substances represent particularly challenging inhibitors in specimens from soil environments, forming complex interactions with both DNA and silica surfaces that reduce binding efficiency and inhibit downstream applications. Washing buffers incorporating polyvinylpyrrolidone (PVP) or bovine serum albumin (BSA) can competitively bind humic acids, reducing their interference with DNA purification and subsequent amplification. The inclusion of an additional wash with buffer containing 20-30% ethanol rather than the standard 70-80% ethanol sometimes improves humic substance removal while retaining bound DNA, though this approach risks increased DNA loss if not carefully optimized. The implementation of environmental DNA extraction principles adapted from soil microbiology provides valuable strategies for addressing the complex inhibitor profiles common in ancient bone specimens.
Elution Strategy Optimization for Maximum Yield
DNA elution from silica beads typically employs low-ionic-strength buffers or water, with standard protocols optimized for modern DNA often resulting in incomplete recovery of ancient DNA due to the stronger binding of short, damaged molecules to silica surfaces. Ancient DNA protocols frequently employ elevated elution temperatures (60-70°C) and extended incubation periods (10-15 minutes) to improve recovery, though these conditions may increase the co-elution of inhibitors if washing was incomplete. Some laboratories implement multiple sequential elutions, pooling the results to maximize yield, though this approach dilutes the final DNA concentration and may be counterproductive for low-concentration extracts. The optimal elution volume represents a balance between concentration requirements and recovery efficiency, with smaller volumes (50-100μL) typically providing higher concentration extracts suitable for most ancient DNA applications.
The chemical composition of the elution buffer influences both yield and downstream compatibility, with TE buffer (10mM Tris, 1mM EDTA, pH 8.0) providing superior DNA stability but potentially interfering with some enzymatic downstream applications. Ancient DNA extracts often benefit from the inclusion of additional stabilizing agents such as DTT or BSA in the elution buffer to protect against oxidative damage and surface adsorption, particularly when working with extremely low DNA quantities. The elution pH requires careful consideration, with slightly alkaline conditions (pH 8.0-8.5) generally providing optimal DNA stability while maintaining efficient disruption of silica-DNA binding. These elution optimization strategies adapted from FFPE extraction protocols demonstrate particular relevance for ancient bone work where DNA quantity is severely limited.
Quality Assessment of Ancient DNA Extracts
Standard DNA quantification methods including spectrophotometry and fluorometry often provide misleading results for ancient bone extracts due to the presence of co-purified contaminants that interfere with accurate measurement. Fluorometric methods using intercalating dyes typically overestimate DNA concentration in ancient extracts due to non-specific binding to degraded DNA and inhibitor molecules, while spectrophotometric approaches suffer from similar issues with contaminant absorption. Quantitative PCR targeting single-copy nuclear loci provides the most accurate quantification for ancient DNA, though this approach consumes valuable extract and may not detect the substantial proportion of damaged molecules incapable of amplification. Many ancient DNA laboratories instead rely on indirect quantification through sequencing library preparation efficiency, though this approach provides retrospective rather than prospective quality assessment.
DNA fragment size distribution analysis provides critical quality information for ancient bone extracts, with the expected profile showing a majority of fragments below 100 base pairs. Automated electrophoresis systems capable of resolving small DNA fragments provide more accurate size distribution data than traditional agarose gels, though the presence of inhibitors can interfere with accurate sizing in some platforms. The degree of DNA damage, particularly cytosine deamination at fragment ends, represents another important quality metric typically assessed through uracil-sensitive and uracil-insensitive enzyme treatments or through analysis of sequence data. These quality assessments inform decisions about downstream application suitability and help identify specimens requiring additional purification or extraction optimization before proceeding to more resource-intensive analyses.
Downstream Application Compatibility
Next-generation sequencing represents the primary downstream application for most ancient bone DNA extracts, with library preparation methods specifically designed to accommodate short, damaged DNA fragments. The single-stranded library construction method has proven particularly valuable for ancient DNA, as it avoids the adapter-dimer formation issues that plague double-stranded approaches when working with extremely short fragments. However, the efficiency of library preparation depends heavily on extract purity, with common ancient bone inhibitors including humic acids and collagen derivatives dramatically reducing ligation efficiency. The compatibility of silica bead-purified ancient DNA with these specialized library construction methods must be validated for each extraction protocol modification, particularly when introducing new washing reagents or buffer components.
Target enrichment approaches including hybridization capture and amplification-based methods enable focused analysis of specific genomic regions from ancient bone extracts, though their success depends on both DNA quality and the absence of inhibitors that interfere with hybridization or polymerase activity. The relatively high DNA input requirements of some capture methods (50-100ng) present challenges for ancient specimens where total endogenous DNA may be limited to picogram quantities. Amplification-based approaches including multiplex PCR face different challenges related to inhibitor sensitivity and the difficulty designing assays that accommodate extensive fragmentation and damage. The implementation of research-grade bone extraction protocols optimized for these specific downstream applications significantly improves the success rate of subsequent genetic analyses.
Case Study: Successful Extraction from Challenging Specimens
A recent analysis of medieval skeletal remains from a waterlogged archaeological site in Northern Europe demonstrates the successful application of modified silica bead protocols to particularly challenging preservation environments. Initial extraction attempts using standard silica bead kits yielded detectable human DNA but with insufficient quality for reliable sequencing, characterized by extreme fragmentation (average 45bp) and substantial inhibitor co-purification. Protocol modifications included extended demineralization in 0.5M EDTA for 72 hours, proteinase K digestion at 0.5mg/mL for 48 hours, and the addition of two extra washes with PVP-containing buffer before final elution in 50μL TE buffer at 65°C for 15 minutes. These modifications increased endogenous DNA content from 0.2% to 3.5% of total DNA while improving average fragment length to 68bp.
The optimized extraction protocol enabled genome-wide capture and sequencing at approximately 0.5X coverage, sufficient for population genetic analysis and kinship determination among the sampled individuals. Interestingly, comparison with extracts from the same specimens using a phenol-chloroform protocol revealed similar endogenous DNA content but higher molecular weight DNA in the organic extracts, suggesting that the silica bead method may selectively recover shorter fragments. This size bias potentially benefits ancient DNA studies where the shortest fragments often show better preservation and less damage, though it may disadvantage applications requiring longer templates. The case study illustrates how systematic optimization of standard silica bead protocols can render even highly degraded, inhibitor-rich specimens amenable to genetic analysis.
Future Directions and Emerging Technologies
The ongoing development of silica bead chemistry specifically designed for ancient DNA applications represents a promising direction for improving extraction efficiency from challenging specimens. Surface-functionalized silica beads with enhanced affinity for short, damaged DNA fragments could dramatically improve recovery from highly degraded samples, while beads with integrated inhibitor capture moieties might address purity issues without requiring additional washing steps. The integration of extraction and library preparation in microfluidic systems could minimize handling-related contamination while reducing reagent consumption, particularly valuable for precious specimens where material is limited. These technological advances, combined with improved understanding of DNA preservation mechanisms in mineralized tissues, will continue to expand the temporal and geographical boundaries of ancient DNA research.
The development of standardized authentication criteria for ancient DNA extracts, including quantitative measures of damage patterns and fragment length distributions, will facilitate comparison across studies and improve reproducibility in the field. Collaborative efforts to establish best practices for silica bead-based ancient DNA extraction, similar to those developed for animal bone extraction in forensic contexts, would provide valuable guidance for laboratories entering the field. As sequencing technologies continue to advance toward requiring less input material, the pressure on extraction efficiency may lessen, though the fundamental challenges of contamination control and inhibitor removal will remain central to successful ancient bone DNA studies. The strategic application of silica bead technology, informed by both empirical optimization and theoretical understanding of DNA-silica interactions, will continue to play a crucial role in unlocking genetic information from the archaeological record.
The extraction of DNA from ancient bone using silica bead kits presents distinct challenges stemming from the degraded nature of the genetic material, complex inhibitor profiles, and persistent contamination risks. Successful application requires substantial modification of standard protocols, with optimization of demineralization, digestion, binding, washing, and elution conditions specifically for ancient specimens. The strategic approaches outlined here, including enhanced binding conditions, rigorous contamination control, and customized inhibitor removal, significantly improve DNA yield and quality while maintaining compatibility with downstream sequencing applications. As ancient DNA research continues to expand our understanding of past populations, the refinement of silica bead extraction methodologies will remain essential for accessing genetic information from increasingly challenging specimens across broader temporal and geographical ranges.
